General Science
The percentage of solar radiation absorbed by all the green plants
The percentage of solar radiation absorbed by all the green plants for the process of photosynthesis is about
- 1%
- 5%
- 8%
- 10%
Which group of organisms are not constituents of a food chain
Which group of organisms are not constituents of a food chain?
- Grass, lion, rabbit, wolf
- Plankton, man, fish, grasshopper
- Wolf, grass, snake, tiger
- Frog, snake, eagle, grass, grasshopper
Select the correct option:
- (i) and (iii)
- (iii) and (iv)
- (ii) and (iii)
- (i) and (iv)
Flow of energy in an ecosystem is always
Flow of energy in an ecosystem is always
- unidirectional
- bidirectional
- multi directional
- no specific direction
In the given food chain, suppose the amount of energy at fourth trophic level is 5 kJ
In the given food chain, suppose the amount of energy at fourth trophic level is 5 kJ, what will be the energy available at the producer level?
Grass → Grasshopper → Frog → Snake → Hawk
- 5 kJ
- 50 kJ
- 500 kJ
- 5000 kJ
In a food chain, the third trophic level is always occupied by
In a food chain, the third trophic level is always occupied by
- carnivores
- herbivores
- decomposers
- producers
To convert an AC generator into DC generator
To convert an AC generator into DC generator
- split-ring type commutator must be used
- slip rings and brushes must be used
- a stronger magnetic field has to be used
- a rectangular wire loop has to be used
The strength of magnetic field inside a long current carrying straight solenoid is
The strength of magnetic field inside a long current carrying straight solenoid is
- more at the ends than at the centre
- minimum in the middle
- same at all points
- found to increase from one end to the other
A constant current flows in a horizontal wire in the plane of the paper
A constant current flows in a horizontal wire in the plane of the paper from east to west as shown in Figure. The direction of magnetic field at a point will be North to South

- directly above the wire
- directly below the wire
- at a point located in the plane of the paper, on the north side of the wire
- at a point located in the plane of the paper, on the south side of the wire
In the arrangement there are two coils wound on a non-conducting cylindrical rod
In the arrangement shown in Figure there are two coils wound on a non-conducting cylindrical rod. Initially the key is not inserted. Then the key is inserted and later removed. Then

- the deflection in the galvanometer remains zero throughout
- there is a momentary deflection in the galvanometer but it dies out shortly and there is no effect when the key is removed
- there are momentary galvanometer deflections that die out shortly; the deflections are in the same direction
- there are momentary galvanometer deflections that die out shortly; the deflections are in opposite directions
Commercial electric motors do not use
Commercial electric motors do not use
- an electromagnet to rotate the armature
- effectively large number of turns of conducting wire in the current carrying coil
- a permanent magnet to rotate the armature
- a soft iron core on which the coil is wound
A uniform magnetic field exists in the plane of paper pointing from left to right
A uniform magnetic field exists in the plane of paper pointing from left to right as shown in Figure. In the field an electron and a proton move as shown. The electron and the proton experience
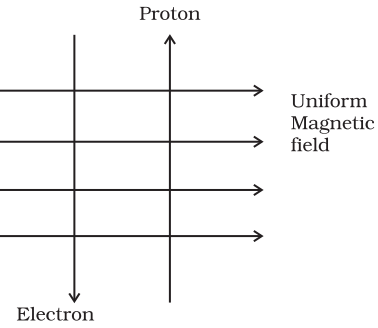
- forces both pointing into the plane of paper
- forces both pointing out of the plane of paper
- forces pointing into the plane of paper and out of the plane of paper, respectively
- force pointing opposite and along the direction of the uniform magnetic field respectively
For a current in a long straight solenoid N- and S-poles are created at the two ends
For a current in a long straight solenoid N- and S-poles are created at the two ends. Among the following statements, the incorrect statement is
- The field lines inside the solenoid are in the form of straight lines which indicates that the magnetic field is the same at all points inside the solenoid
- The strong magnetic field produced inside the solenoid can be used to magnetise a piece of magnetic material like soft iron, when placed inside the coil
- The pattern of the magnetic field associated with the solenoid is different from the pattern of the magnetic field around a bar magnet
- The N- and S-poles exchange position when the direction of current through the solenoid is reversed
A circular loop placed in a plane perpendicular to the plane of paper
A circular loop placed in a plane perpendicular to the plane of paper carries a current when the key is ON. The current as seen from points A and B (in the plane of paper and on the axis of the coil) is anti clockwise and clockwise respectively. The magnetic field lines point from B to A. The N-pole of the resultant magnet is on the face close to

- A
- B
- A if the current is small, and B if the current is large
- B if the current is small and A if the current is large
If the key in the arrangement is taken out and magnetic field lines are drawn
If the key in the arrangement is taken out (the circuit is made open) and magnetic field lines are drawn over the horizontal plane ABCD, the lines are

- concentric circles
- elliptical in shape
- straight lines parallel to each other
- concentric circles near the point O but of elliptical shapes as we go away from it
Two resistors of resistance 2 Ω and 4 Ω when connected to a battery
Two resistors of resistance 2 Ω and 4 Ω when connected to a battery will have
- same current flowing through them when connected in parallel
- same current flowing through them when connected in series
- same potential difference across them when connected in series
- different potential difference across them when connected in parallel
An electric kettle consumes 1 kW of electric power when operated at 220 V
An electric kettle consumes 1 kW of electric power when operated at 220 V. A fuse wire of what rating must be used for it?
- 1 A
- 2 A
- 4 A
- 5 A
In an electrical circuit two resistors of 2 Ω and 4 Ω respectively
In an electrical circuit two resistors of 2 Ω and 4 Ω respectively are connected in series to a 6 V battery. The heat dissipated by the 4 Ω resistor in 5 s will be
- 5 J
- 10 J
- 20 J
- 30 J
In an electrical circuit three incandescent bulbs A, B and C of rating
In an electrical circuit three incandescent bulbs A, B and C of rating 40 W, 60 W and 100 W respectively are connected in parallel to an electric source. Which of the following is likely to happen regarding their brightness?
- Brightness of all the bulbs will be the same
- Brightness of bulb A will be the maximum
- Brightness of bulb B will be more than that of A
- Brightness of bulb C will be less than that of B
If the current I through a resistor is increased by 100%
If the current I through a resistor is increased by 100% (assume that temperature remains unchanged), the increase in power dissipated will be
- 100%
- 200%
- 300%
- 400%
A cylindrical conductor of length l and uniform area of cross-section
A cylindrical conductor of length l and uniform area of cross-section A has resistance R. Another conductor of length 2l and resistance R of the same material has area of cross section
- A/2
- 3A/2
- 2A
- 3A
What is the maximum resistance which can be made using five resistors
What is the maximum resistance which can be made using five resistors each of 1/5 Ω?
- 1/5 Ω
- 10 Ω
- 5 Ω
- 1 Ω
A current of 1 A is drawn by a filament of an electric bulb
A current of 1 A is drawn by a filament of an electric bulb. Number of electrons passing through a cross section of the filament in 16 seconds would be roughly
- 1020
- 1016
- 1018
- 1023
Electrical resistivity of a given metallic wire depends upon
Electrical resistivity of a given metallic wire depends upon
- its length
- its thickness
- its shape
- nature of the material
In the following circuits, heat produced in the resistor or combination
In the following circuits, heat produced in the resistor or combination of resistors connected to a 12 V battery will be

- same in all the cases
- minimum in case (i)
- maximum in case (ii)
- maximum in case (iii)
The focal length of the eye lens increases when eye muscles
The focal length of the eye lens increases when eye muscles
- are relaxed and lens becomes thinner
- contract and lens becomes thicker
- are relaxed and lens becomes thicker
- contract and lens becomes thinner
When light rays enter the eye, most of the refraction occurs at the
When light rays enter the eye, most of the refraction occurs at the
- crystalline lens
- outer surface of the cornea
- iris
- pupil
The bluish colour of water in deep sea is due to
The bluish colour of water in deep sea is due to
- the presence of algae and other plants found in water
- reflection of sky in water
- scattering of light
- absorption of light by the sea
Which phenomena contributes significantly to the reddish appearance of the sun
Which of the following phenomena contributes significantly to the reddish appearance of the sun at sunrise or sunset?
- Dispersion of light
- Scattering of light
- Total internal reflection of light
- Reflection of light from the earth
The clear sky appears blue because
The clear sky appears blue because
- blue light gets absorbed in the atmosphere
- ultraviolet radiations are absorbed in the atmosphere
- violet and blue lights get scattered more than lights of all other colours by the atmosphere
- light of all other colours is scattered more than the violet and blue colour lights by the atmosphere
Twinkling of stars is due to atmospheric
Twinkling of stars is due to atmospheric
- dispersion of light by water droplets
- refraction of light by different layers of varying refractive indices
- scattering of light by dust particles
- internal reflection of light by clouds
Which phenomena of light are involved in the formation of a rainbow
Which of the following phenomena of light are involved in the formation of a rainbow?
- Reflection, refraction and dispersion
- Refraction, dispersion and total internal reflection
- Refraction, dispersion and internal reflection
- Dispersion, scattering and total internal reflection
At noon the sun appears white as
At noon the sun appears white as
- light is least scattered
- all the colours of the white light are scattered away
- blue colour is scattered the most
- red colour is scattered the most
A person cannot see distinctly objects kept beyond 2 m
A person cannot see distinctly objects kept beyond 2 m. This defect can be corrected by using a lens of power
- + 0.5 D
- – 0.5 D
- + 0.2 D
- – 0.2 D
A full length image of a distant tall building
A full length image of a distant tall building can definitely be seen by using
- a concave mirror
- a convex mirror
- a plane mirror
- both concave as well as plane mirror
Rays from Sun converge at a point 15 cm in front of a concave mirror
Rays from Sun converge at a point 15 cm in front of a concave mirror. Where should an object be placed so that size of its image is equal to the size of the object?
- 15 cm in front of the mirror
- 30 cm in front of the mirror
- between 15 cm and 30 cm in front of the mirror
- more than 30 cm in front of the mirror
Magnification produced by a rear view mirror fitted in vehicles
Magnification produced by a rear view mirror fitted in vehicles
- is less than one
- is more than one
- is equal to one
- can be more than or less than one depending upon the position of the object in front of it
A light ray enters from medium A to medium B
A light ray enters from medium A to medium B as shown in Figure.

The refractive index of medium B relative to A will be
- greater than unity
- less than unity
- equal to unity
- zero
A 10 mm long awl pin is placed vertically in front of a concave mirror
A 10 mm long awl pin is placed vertically in front of a concave mirror. A 5 mm long image of the awl pin is formed at 30 cm in front of the mirror. The focal length of this mirror is
- – 30 cm
- – 20 cm
- – 40 cm
- – 60 cm
Which can make a parallel beam of light when light from a point source
Which of the following can make a parallel beam of light when light from a point source is incident on it?
- Concave mirror as well as convex lens
- Convex mirror as well as concave lens
- Two plane mirrors placed at 90° to each other
- Concave mirror as well as concave lens
The theory of evolution of species by natural selection was given by
The theory of evolution of species by natural selection was given by
- Mendel
- Darwin
- Morgan
- Lamarck
In peas, a pure tall plant (TT) is crossed with a short plant (tt)
In peas, a pure tall plant (TT) is crossed with a short plant (tt). The ratio of pure tall plants to short plants in F2 is
- 1 : 3
- 3 : 1
- 1 : 1
- 2 : 1
A trait in an organism is influenced by
A trait in an organism is influenced by
- paternal DNA only
- maternal DNA only
- both maternal and paternal DNA
- neither by paternal nor by maternal DNA
A zygote which has an X-chromosome inherited from the father
A zygote which has an X-chromosome inherited from the father will develop into a
- boy
- girl
- X- chromosome does not determine the sex of a child
- either boy or girl
If a round, green seeded pea plant (RR yy) is crossed with wrinkled
If a round, green seeded pea plant (RR yy) is crossed with wrinkled, yellow seeded pea plant, (rr YY) the seeds produced in F1 generation are
- round and yellow
- round and green
- wrinkled and green
- wrinkled and yellow
A cross between a tall plant (TT) and short pea plant (tt) resulted
A cross between a tall plant (TT) and short pea plant (tt) resulted in progeny that were all tall plants because
- tallness is the dominant trait
- shortness is the dominant trait
- tallness is the recessive trait
- height of pea plant is not governed by gene ‘T’ or ‘t’
Two pink coloured flowers on crossing resulted in 1 red, 2 pink
Two pink coloured flowers on crossing resulted in 1 red, 2 pink and 1 white flower progeny. The nature of the cross will be
- double fertilisation
- self pollination
- cross fertilisation
- no fertilisation
Which among the following diseases is not sexually transmitted
Which among the following diseases is not sexually transmitted?
- Syphillis
- Hepatitis
- HIV - AIDS
- Gonorrhoea
The correct sequence of organs in the male reproductive system
The correct sequence of organs in the male reproductive system for transport of sperms is
- testis → vasdeferens → urethra
- testis → ureter → urethra
- testis → urethra → ureter
- testis → vasdeferens → ureter
Length of pollen tube depends on the distance between
Length of pollen tube depends on the distance between
- pollen grain and upper surface of stigma
- pollen grain on upper surface of stigma and ovule
- pollen grain in anther and upper surface of stigma
- upper surface of stigma and lower part of style
Vegetative propagation refers to formation of new plants from
Vegetative propagation refers to formation of new plants from
- stem, roots and flowers
- stem, roots and leaves
- stem, flowers and fruits
- stem, leaves and flowers
Page 1 of 7